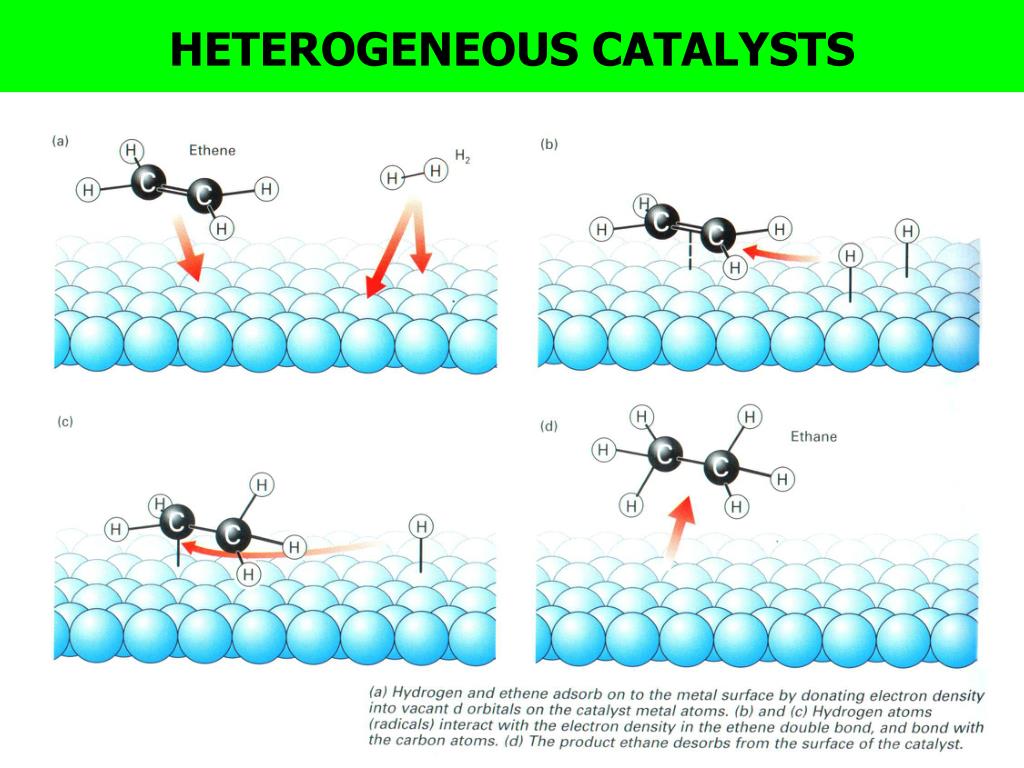
Catalyst Gas Examples . gaseous and liquid catalysts are commonly used in their pure form or in combination with suitable carriers or solvents; because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and the reverse reactions by the. the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; Most examples of heterogeneous catalysis go through the same stages:. Hydrolysis of an ester like ethyl acetate (ch 3 cooc 2 h 5) in the presence of an acid catalyst (aq. this page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they. typical examples involve a solid catalyst with the reactants as either liquids or gases. Adding a drop of blood to a solution of 30% hydrogen.
from www.slideserve.com
because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and the reverse reactions by the. Most examples of heterogeneous catalysis go through the same stages:. the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; Hydrolysis of an ester like ethyl acetate (ch 3 cooc 2 h 5) in the presence of an acid catalyst (aq. gaseous and liquid catalysts are commonly used in their pure form or in combination with suitable carriers or solvents; this page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they. typical examples involve a solid catalyst with the reactants as either liquids or gases. Adding a drop of blood to a solution of 30% hydrogen.
PPT Starter 1)Definition of catalysts 2) Difference between
Catalyst Gas Examples this page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they. the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; gaseous and liquid catalysts are commonly used in their pure form or in combination with suitable carriers or solvents; Most examples of heterogeneous catalysis go through the same stages:. typical examples involve a solid catalyst with the reactants as either liquids or gases. because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and the reverse reactions by the. this page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they. Hydrolysis of an ester like ethyl acetate (ch 3 cooc 2 h 5) in the presence of an acid catalyst (aq. Adding a drop of blood to a solution of 30% hydrogen.
From www.slideserve.com
PPT Catalysts PowerPoint Presentation, free download ID2568751 Catalyst Gas Examples typical examples involve a solid catalyst with the reactants as either liquids or gases. this page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they. Hydrolysis of an ester like ethyl acetate (ch 3 cooc 2 h 5) in the presence of an acid catalyst (aq.. Catalyst Gas Examples.
From slidetodoc.com
Heterogeneous Catalysis Solid State Physics 141 A Dohyung Catalyst Gas Examples Most examples of heterogeneous catalysis go through the same stages:. Hydrolysis of an ester like ethyl acetate (ch 3 cooc 2 h 5) in the presence of an acid catalyst (aq. this page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they. because a catalyst decreases. Catalyst Gas Examples.
From www.youtube.com
Identifying catalysts in a reaction YouTube Catalyst Gas Examples because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and the reverse reactions by the. the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; this page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples. Catalyst Gas Examples.
From www.slideserve.com
PPT Industrial catalysis PowerPoint Presentation, free download ID Catalyst Gas Examples this page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they. the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; Hydrolysis of an ester like ethyl acetate (ch 3 cooc 2 h 5) in the presence of an acid. Catalyst Gas Examples.
From www.britannica.com
Catalyst Examples, Definition, & Facts Britannica Catalyst Gas Examples Adding a drop of blood to a solution of 30% hydrogen. because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and the reverse reactions by the. gaseous and liquid catalysts are commonly used in their pure form or in combination with suitable carriers or solvents; this. Catalyst Gas Examples.
From dxomuorny.blob.core.windows.net
Catalyst In Chemistry Examples at Alton Stonge blog Catalyst Gas Examples gaseous and liquid catalysts are commonly used in their pure form or in combination with suitable carriers or solvents; the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and the. Catalyst Gas Examples.
From titaniumoxide.blogspot.com
The examples of catalysis Catalyst Gas Examples gaseous and liquid catalysts are commonly used in their pure form or in combination with suitable carriers or solvents; Adding a drop of blood to a solution of 30% hydrogen. this page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they. Hydrolysis of an ester like. Catalyst Gas Examples.
From www.dreamstime.com
Catalyst Surface with Catalytic Reaction Stock Vector Illustration of Catalyst Gas Examples typical examples involve a solid catalyst with the reactants as either liquids or gases. this page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they. because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward. Catalyst Gas Examples.
From www.researchgate.net
Homogeneous vs heterogeneous catalysts. Design of heterogeneous Catalyst Gas Examples gaseous and liquid catalysts are commonly used in their pure form or in combination with suitable carriers or solvents; this page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they. Hydrolysis of an ester like ethyl acetate (ch 3 cooc 2 h 5) in the presence. Catalyst Gas Examples.
From www.aecc.eu
AECC Catalysts From oxidation catalysts to threeway catalysts Catalyst Gas Examples this page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they. gaseous and liquid catalysts are commonly used in their pure form or in combination with suitable carriers or solvents; Hydrolysis of an ester like ethyl acetate (ch 3 cooc 2 h 5) in the presence. Catalyst Gas Examples.
From www.ck12.org
Catalysts Example 1 ( Video ) Chemistry CK12 Foundation Catalyst Gas Examples Hydrolysis of an ester like ethyl acetate (ch 3 cooc 2 h 5) in the presence of an acid catalyst (aq. this page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they. Adding a drop of blood to a solution of 30% hydrogen. because a catalyst. Catalyst Gas Examples.
From www.dreamstime.com
An Abstract Cutaway Diagram of a Catalyst with Chemical Elements at the Catalyst Gas Examples the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; gaseous and liquid catalysts are commonly used in their pure form or in combination with suitable carriers or solvents; Adding a drop of blood to a solution of 30% hydrogen. this page looks at the the different types of catalyst (heterogeneous. Catalyst Gas Examples.
From www.slideserve.com
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint Catalyst Gas Examples this page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they. the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; typical examples involve a solid catalyst with the reactants as either liquids or gases. gaseous and liquid. Catalyst Gas Examples.
From www.britannica.com
fuel cell summary Britannica Catalyst Gas Examples the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; Adding a drop of blood to a solution of 30% hydrogen. this page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they. gaseous and liquid catalysts are commonly used. Catalyst Gas Examples.
From www.pinterest.com
Homogeneous Catalyst Easy Science Ap chemistry, Chemical equation Catalyst Gas Examples Hydrolysis of an ester like ethyl acetate (ch 3 cooc 2 h 5) in the presence of an acid catalyst (aq. because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and the reverse reactions by the. the most effective catalyst of all is the enzyme catalase, present. Catalyst Gas Examples.
From schematicdiagramyakuza.z13.web.core.windows.net
Catalyst Diagram Chemistry Catalyst Gas Examples typical examples involve a solid catalyst with the reactants as either liquids or gases. the most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; Hydrolysis of an ester like ethyl acetate (ch 3 cooc 2 h 5) in the presence of an acid catalyst (aq. Adding a drop of blood to a. Catalyst Gas Examples.
From www.degruyter.com
Mesoporous catalysts for catalytic oxidation of volatile organic Catalyst Gas Examples typical examples involve a solid catalyst with the reactants as either liquids or gases. gaseous and liquid catalysts are commonly used in their pure form or in combination with suitable carriers or solvents; Most examples of heterogeneous catalysis go through the same stages:. the most effective catalyst of all is the enzyme catalase, present in blood and. Catalyst Gas Examples.
From www.slideserve.com
PPT Starter 1)Definition of catalysts 2) Difference between Catalyst Gas Examples typical examples involve a solid catalyst with the reactants as either liquids or gases. because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and the reverse reactions by the. Adding a drop of blood to a solution of 30% hydrogen. this page looks at the the. Catalyst Gas Examples.